Background. Vididencel is an allogeneic leukemia-derived dendritic cell vaccine being developed as maintenance treatment in AML patients. Intradermal vaccination using vididencel is known to induce a local skin reaction, with local antigen processing by skin dendritic cells and direct presentation to T cells as well as dendritic cells, crawling out of the skin and presentation of the antigen in the draining lymph nodes for a full-blown immune response. It was previously shown that vididencel is able to induce an influx of immune cells in the skin using standard immunohistochemistry (van de Loosdrecht, 2018). In a phase 2 study vididencel was investigated as AML maintenance treatment after induction of CR1 with chemotherapy (ADVANCE-II, Clintrials.gov: NCT03697707). Using imaging mass cytometry up to 43 markers can be assessed in the same tissue section, to allow in depth analysis of the immune cells on a single cell level infiltrating in the dermis at the injection site.
Methods. AML patients, in first complete remission with measurable residual disease (MRD +) and not planned for HSCT at inclusion, received 4 biweekly doses of vididencel followed by 2 booster doses at week 14 and 18. Skin biopsies of the injection site were taken 2 days after the 1 st, 4 th and 6 th vaccination. Control samples were collected from unvaccinated/unaffected skin in two patients and from healthy controls. Samples were fixed and embedded in paraffin, and stained by standard immunohistochemistry for immune cell markers like CD4, CD8 and CD68 in the central lab. For patients treated at Haukeland University Hospital (N=6), tissue sections were prepared, mounted and stained with the panel of 29 antibodies labelled with heavy metal isotopes for use in the Hyperion mass cytometry imaging. For each sample a region of interest (ROI) of 2.8mm 2 was scanned based on the localization of the immune infiltrates, guided by standard H&E staining from neighboring slides.
Results
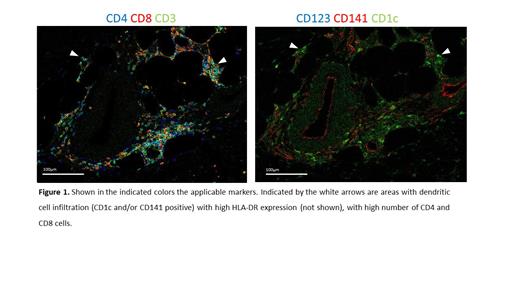
In 88.5% of patients injection site reactions were reported in this phase 2 study, characterized by redness, swelling and warmth at the site of intradermal injection, which were mild (maximum grade 1 or 2), and were of short duration (generally resolved within 12 days). Skin biopsies of a subset of 6 patients were analyzed using Hyperion. In standard single stain immunohistochemistry a large influx of CD4, CD8 and CD68 cells was observed in contrast to normal skin. Imaging mass cytometry confirmed these dense immune infiltrations. Using additional markers to characterize monocytes, dendritic cells and T-cells revealed that these cells are in close proximity and suggestive for cell-to-cell interaction (Figure 1). At sites of dense immune infiltration, influx of GranzymeB expressing CD8 T-cells and CD38 positive CD4 T-cells were observed as well as CD1c+ or CD141+ dendritic cells with high HLA-DR expression. These T-cells and dendritic cells are in close contact suggestive for patients' dendritic cells migrating from blood to skin, inducing a local immune response.
Conclusion/discussion.
Vaccination with vididencel triggered an inflammatory skin reaction in the majority of patients, with redness, warmth and swelling, of short duration, indicative for an active immune response at the site of injection. Skin biopsies using standard immunohistochemistry showed influx of activated CD4 and CD8 T cells as well as antigen presenting cell, including dendritic cells.Extended analysis with multiparameter imaging mass cytometry showed specific immune cell subsets infiltrating in these site of injection sites. The observed co-localization of the different immune subsets following vaccination supports the hypothesis of local antigen capturing by blood derived and local antigen presenting cells (Zuo et al, 2021). The local immune response may ultimately leads to a systemic immune response and disease control.
Disclosures
Van Zeeburg:Mendus AB: Current Employment. Rovers:Mendus AB: Current Employment, Current equity holder in publicly-traded company. Van de Loosdrecht:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Gjertsen:Sanofi: Consultancy; Pfizer: Consultancy, Research Funding; Otsuka: Consultancy; Novartis: Consultancy, Research Funding; Mendus AB: Consultancy, Research Funding; InCyte: Consultancy; Immedica: Consultancy; GreinDX: Consultancy; BerGenBio: Consultancy; Coegin: Consultancy; in Alden Cancer Therapy AS: Current holder of stock options in a privately-held company; KinN Therapeutics AS: Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal